The Stakes Are High in the Life Sciences Location Game
Savvy leadership will recognize current trends and options and carefully analyze established criteria when making the company's next location decision.
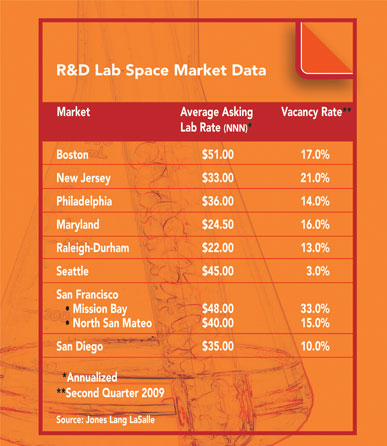
Biotech Location Guide 2009
Cost-Saving Trends
Like many life sciences companies today, your company might be under tremendous financial pressure to cut costs and consolidate facilities due to factors such as the lack of a viable R&D pipeline for future drugs; an excess of facilities due to the acquisition of other biotech and pharmaceutical companies; or because your existing drugs are coming off of patents. In the past, life sciences companies were focused on increasing top-line revenue but, today, cutting bottom-line costs is being aggressively pursued.
Consequently, many life sciences companies are rationalizing their overall real estate portfolio in an effort to cut fixed costs. In many cases, consolidating facilities or deferring capital projects is becoming commonplace; however, four trends, in particular, are gaining significant and noteworthy traction, and may be considered viable options:
An increasing number of life sciences companies are selling their facilities to a third-party service provider, entering into service agreements, and then contracting back the exact scientific services that the life sciences company was conducting at the facility. The life sciences company reduces its fixed costs and can then focus on its core expertise, while the third-party provider can take advantage of excess capacity in the facility and drive more revenue through these facilities.
Another trend gaining traction is the outsourcing of facilities management to a third-party provider so the life sciences company can focus on its core area of expertise and use the services of best-in-class facilities management professionals to manage the real estate. The life sciences company reduces fixed costs while concurrently receiving the benefits of leading-edge thought leadership on efficiently and effectively running its facilities.
Traditionally, laboratories are very heavy users of energy because a large amount of energy is consumed in the heating and cooling of a lab. The proactive management of utilities with a focus on energy reduction and efficiency is becoming increasingly important. For example, in the past, the ventilation rate for a typical chemistry lab would be 12-14 air changes per hour. In many labs, this rate has been greatly reduced in order to cut costs, sometimes to as low as six air changes per hour.
Other emerging technologies are increasingly focusing on issues concerning energy reduction and efficiency. For instance, fume hoods (whose purpose is to control users' exposure to toxic or flammable gases or vapors) are normally closed manually. Occupancy sensors have now been created that will automatically close these fume hood sashes after a predetermined length of time. In fact, some companies are giving scientists and researchers an energy budget related to fume hood use in an effort to "incentivize" the way researchers use their space.
A fourth cost savings measure is to design new labs to be easily reconfigured so that when one group of scientists is done with the lab, a new group can move in within hours or weeks - instead of the months it would take to reconfigure a new lab to meet the exacting requirements of a scientific work environment. These "plug-and-play labs of the future" are easily adaptable and much less costly than traditional labs.
Project Announcements
Taiwan-Based Bora Pharmaceuticals Expands Baltimore, Maryland Operations
11/13/2025
Aqua Membranes Plans Knoxville, Tennessee, Manufacturing Operations
11/13/2025
Eli Lilly and Company Expands Carolina, Puerto Rico, Manufacturing Operations
11/12/2025
ACG Plans Conyers, Georgia, Manufacturing Operations
10/30/2025
Merck & Co. Expands Elkton, Virginia, Manufacturing Operations
10/28/2025
Cardinal Biologicals Expands Cape Girardeau, Missouri, Operations
10/27/2025
Most Read
-
2025’s Top States for Business: How the Winners Are Outpacing the Rest
Q3 2025
-
Rethinking Local Governments Through Consolidation and Choice
Q3 2025
-
The Compliance Reckoning Is Here
Q3 2025
-
Around the Horn: Data Center Supply Chains — What's Next?
Q3 2025
-
First Person: Filter King’s Expansion Playbook
Q3 2025
-
Rethinking Auto Site Strategy in the Age of Tariffs and Powertrain Shifts
Q3 2025
-
Lead with Facts, Land the Deal
Q3 2025